The world needs a new approach to treating serious pain
Tris is working with urgency to develop a pipeline of differentiated compounds based on ground-breaking advances in the evolution of pain biology modulation. Our lead investigational compound, cebranopadol, is the world’s first dual nociceptin/orphanin FQ peptide (NOP) receptor and µ-opioid peptide (MOP) receptor (dual-NMR) agonist and has the potential to offer gold-standard efficacy with minimized risk of detrimental side effects.
In Q1 2025, Tris shared positive results of its two registrational acute pain trials in patients following abdominoplasty and bunionectomy surgeries. These data are foundational to discussions with regulators in anticipation of NDA submission.
Breaking the mold in pain biology: effective pain management
with a strong safety profile
At Tris, we are pioneering a fundamentally different approach to pain biology modulation: the dual-NMR agonist.
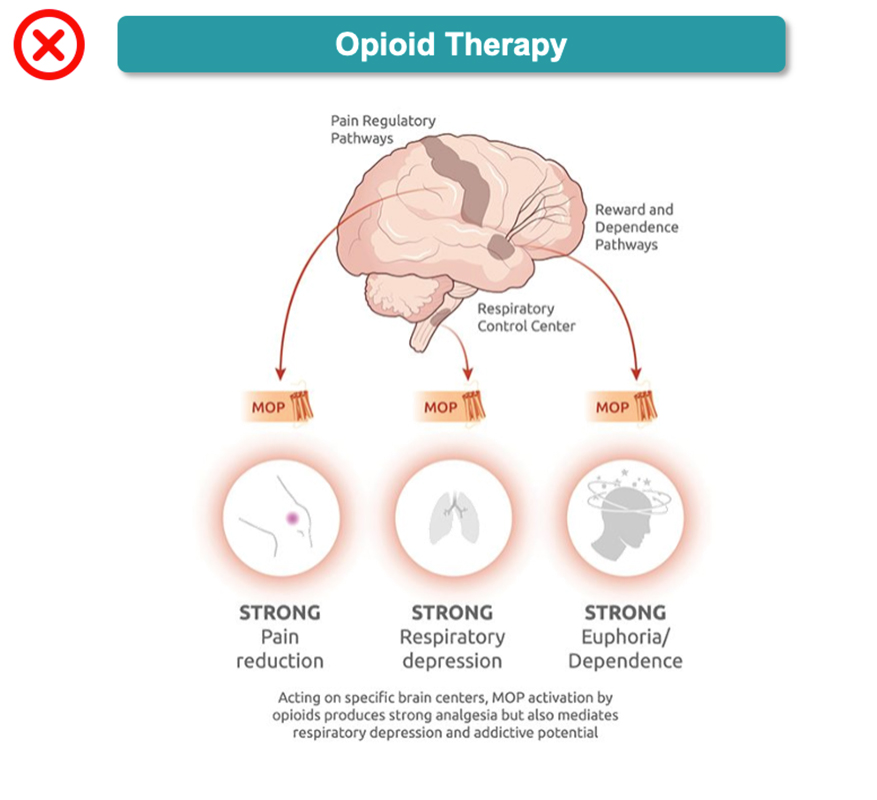
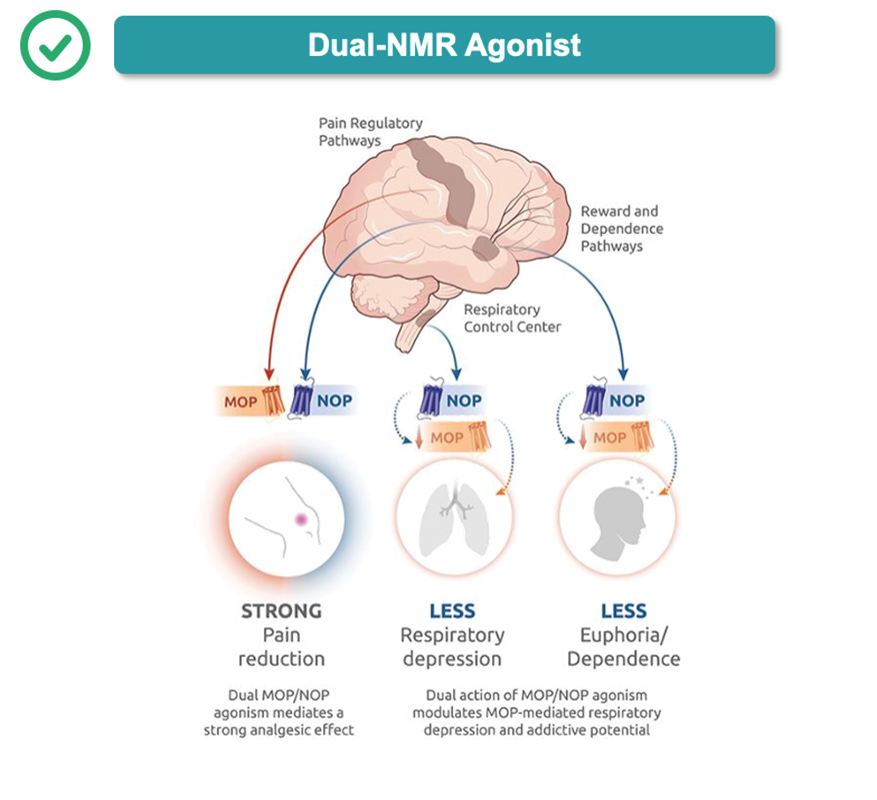

Dual-NMR agonist compounds have the potential to deliver gold-standard pain relief with a strong safety profile because they mimic the body’s natural pain-modulation processes. By leveraging the body’s innate coactivation of NOP and MOP, dual-NMR agonists synergize the analgesic and safety characteristics of the NOP receptor with the analgesic advantages of the MOP receptor. Our investigational dual-NMR agonist, cebranopadol, has been well-characterized, studied in approximately 2,200 patients across 33 trials, including two pivotal Phase 3 trials in acute pain. Tris also plans to conduct Phase 3 trials in chronic pain.

Research into the potential safety advantages of the dual-NMR mechanism is being supported by the NIH through a significant award
Because of cebranopadol’s novel approach to pain modulation, we have generated promising preclinical data for the treatment of addiction. Based on these findings, Tris has been awarded up to $16.6M over five years from the National Institute on Drug Abuse (NIDA), part of the National Institutes of Health (NIH), to evaluate cebranopadol for the treatment of opioid and substance use disorder.
This research is supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number UG3DA059285. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Frequently Asked Questions
Cebranopadol is an investigational first-in-class analgesic in clinical development for the treatment of moderate-to-severe pain. Its novel mechanism has the potential to transform pain treatment so that patients can obtain relief with minimized risk of significant side effects. To date, cebranopadol has been studied in over 2,000 patients across 32 clinical trials and has demonstrated a promising efficacy and safety profile.
Cebranopadol’s novel mechanism of action targets two key receptors, the nociceptin/orphanin FQ peptide (NOP) and µ-opioid peptide (MOP) receptors. These receptors play complementary and distinct roles to modulate pain biology, synergizing the analgesic and safety characteristics of the NOP receptor with the analgesic advantages of the MOP receptor
The human body utilizes sophisticated pain modulation pathways with key receptors that receive and transmit pain signals. Two of these key receptors are the NOP and the MOP receptors, which have co-evolved to work together to regulate pain.
Current treatments for moderate-to-severe pain involving opioids have well-described safety problems that may largely derive from singularly targeting the MOP receptor. Activation of the NOP receptor has demonstrated distinct and complementary effects to MOP, resulting in less respiratory depression, less stimulation of the brain reward pathway and less euphoria (Ciccocioppo 2000; Di Giannuario 2000; Linz 2017). This coactivation of NOP and MOP receptors (“dual-NMR”) is the key to cebranopadol’s unique mechanism of action.
In clinical trials to date, cebranopadol has exhibited efficacy comparable to opioids and has the potential to address an unmet medical need for patients suffering from pain. With its novel, dual-NMR mechanism of action, cebranopadol offers the potential to effectively treat moderate-to-severe pain with minimized risk of significant side effects, especially dependence, addiction and respiratory depression.
Two cebranopadol human abuse potential studies conducted head-to head against Class-II and Class-IV opioids have demonstrated that cebranopadol-treated subjects, even at supratherapeutic doses, performed much better as measured by drug-liking. Moreover, data to date suggest that cebranopadol does not produce meaningful physical dependence. In studies of subjects treated with cebranopadol for up to 14 weeks, abrupt discontinuation of cebranopadol did not require tapering or cause patients to exhibit clinically relevant withdrawal symptoms.
Cebranopadol’s novel mechanism of action also lends promise in treating patients with substance use disorders. Tris plans to continue to evaluate cebranopadol to treat these patients and has been provided a $16.6 million grant by the National Institutes of Health (NIH) to study the drug for opioid use disorder.
Tris anticipates submitting an NDA for acute pain in 2025.
In Q1 2025, Tris shared positive results of its two registrational acute pain trials in patients following abdominoplasty and bunionectomy surgeries. These data are foundational to discussions with regulators in anticipation of NDA submission.
Tris anticipates conducting clinical trials in chronic pain conditions including neuropathic pain. Cebranopadol has been granted Fast Track Designation for chronic low back pain.
For more information on cebranopadol, view our clinical publications, press releases and notable news coverage.
